Compound That Produces Hydronium Ions in Water
When an acid reacts with an active metal a. When hydrochloric acid HCl dissolves in water it ionizes splitting up into hydrogen H and chlorine Cl- ions.

Litmus Is A Water Soluble Mixture Of Different Dyes Extracted From Lichens Especially Roccella Tinctoria It Is Often Absorbed Onto Fi Lichen Fungi Old Things
Hydronium ion or H3O can be produced by adding acids to water H2O.

. Water is a weak solution of hydronium hydroxide - there is an equilibrium 2H 2 O H 3 O OH in combination with solvation of the resulting hydronium ions. Chemically an acid is any substance that produces hydronium ions H3O when dissolved in water. Some compounds which are acidic and produces hydrogen ions in solutions are HCl H 2 S O 4 H N O 3 C H 3 C O O H etc.
A compound that produces hydroxide ions when dissolved in water. Equal in acid strength to acetic acid. An Arrhenius acid releases hydrogen ions in water which combine with water to produce hydronium ions H3O.
Ionic compounds produce ions in solution by. An acid ionizes in water. In chemistry hydronium hydroxonium in traditional British English is the common name for the aqueous cation H3O the type of oxonium ion produced by protonation of water.
H3O ions which form when an acid dissolves in water and H ions interact with water. 23 Acids Bases and Salts. A chemical reaction between an acid and a base.
It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water as Arrhenius acid molecules in solution. An ionic compound formed when an acid reacts with a base. An acid has a bitter taste.
Pure water has a low electrical conductivity which increases with the dissolution of a small amount of ionic material such as common salt. The Patton-Reeder indicator is a suitable indicator in this case as it produces a clear colour change from pinkred to blue in the pH range of 12 14. All magnesium ions precipitate as magnesium hydroxide before the indicator is added.
Hence acids are the compounds that produce hydrogen ions in solutions. A measure of the concentration of hydronium ions in the solution using a scale ranging from 0-14 with 0 being the most acidic and 14 being the most basic. HCN is classified as a weak acid in water.
The process by which some molecular compounds dissolve in water to form ions is. A substance that produces a hydronium ion when dissolved in water is called aan A. 100 of the maximum of possible hydronium ions.
Chemical Formula usually HXX monatomic or polyatomic. Hydrochloric acid HC added to water can produce hydronium cations and chloride Cl- anions. Bases change the ___ of acid-base indicators.
Higher than the boiling point of the solvent. NaOH Na OH and similarly in water the acid hydrogen chloride forms hydronium and chloride ions. Uh well Hydronium is H30 soooo if Water is Neutral then H30 is an Acid cause of the.
Any substance that produces hydrogen ions H in a water solution. A stronger acid than the acetic acid. A weaker acid than acetic acid.
Solution containing ions that react with added acids. Bases ___ fats and oils. 32 Are Oxoacids ionic or covalent.
A compound that produces hydronium ions H3O when dissolved in water. What is a compound. Acids are molecular compounds that ionize in water to produce ____ ions.
Forms hydronium ions in water is a characteristic of an acid base or both. The hydronium ions concentration increases. 33 How many more H ions does a solution of pH of 4 have than a solution of pH of 7.
This is because the water molecule readily takes up the liberated H ion from the acids and forms a hydronium ion. The reaction of hydronium ions and hydroxide ions to form water molecules is called. What is the term for.
Compound formed when negative ions from an acids combine with. An acid produces hydronium ions in water. Bases are _____ compounds that dissociate in water to produce hydroxide ions.
The metal forms anions. A compound that produces hydrogen ions when dissolved in water. Hydrogen gas is produced.
An acid is a compound that dissolves in water to make a particular kind of solution. Carbon dioxide gas is produced. Acid has a bitter taste.
Electrical conductivity and electrolysis. This means that it produces_____. 31 Which of the following is a molecular compound that produces H ions when dissolved in water h2so4.
A measure of the hydronium ion concentration of. The boiling point of a liquid solution is. H3O ions which form when an acid dissolves in water and H ions interact with water.

Hydration The Hydronium Ion Ions In Solutions Mcat Content
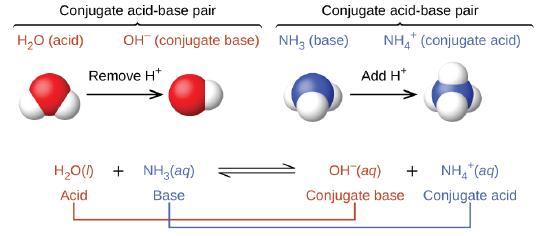
15 3 Definitions Of Acids And Bases Chemistry Libretexts

9 2 What Are Acids An Acid Is A Compound That Dissolves In Water To Make A Particular Kind Of Solution Chemically An Acid Is Any Substance That Produces Ppt Download
Comments
Post a Comment